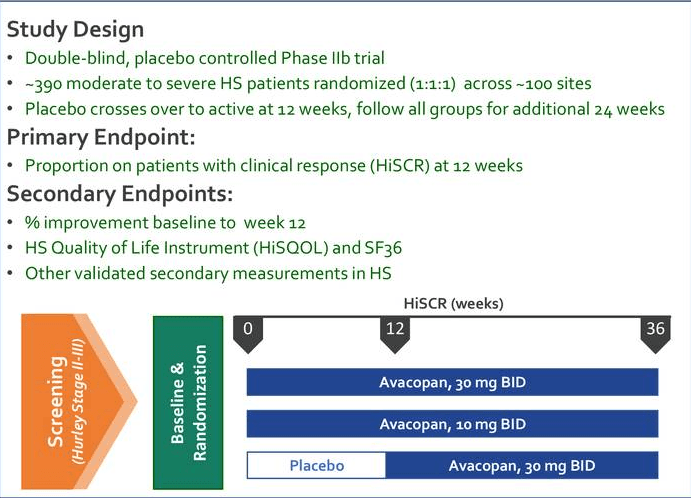
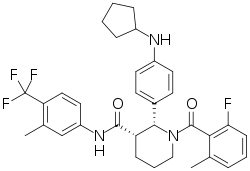
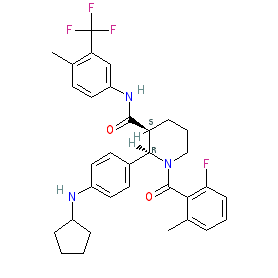
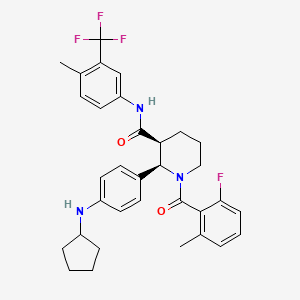
ChemoCentryx Announces Withdrawal of Phase II-Based Conditional Marketing Authorisation (CMA) Application for ANCA-Associated Vasculitis in Europe, Phase III Advocate Trial Data Release Planned for Q4 2019 | Business Wire

La vía alternativa del complemento abre nuevas opciones terapéuticas para la vasculitis asociada a ANCA