EAHP survey on the future potential of electronic product information (ePI) | European Association of Hospital Pharmacists

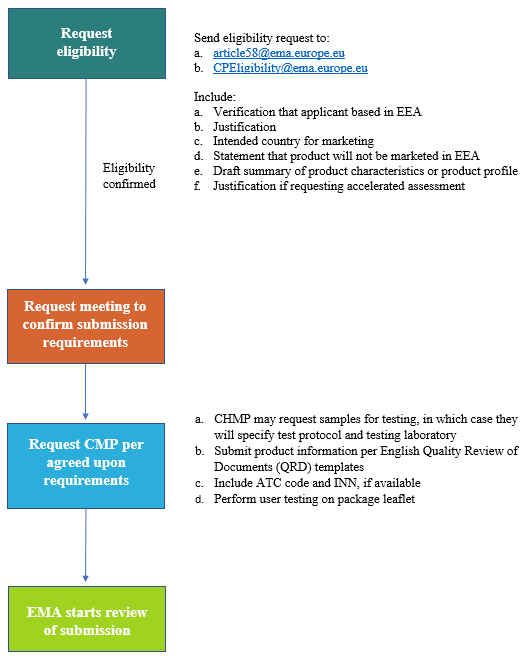
PDF) Consultation on QRD Recommendations on Pack Design and Labelling - response to consultation (EMA/275297/2010) | Theo Raynor - Academia.edu

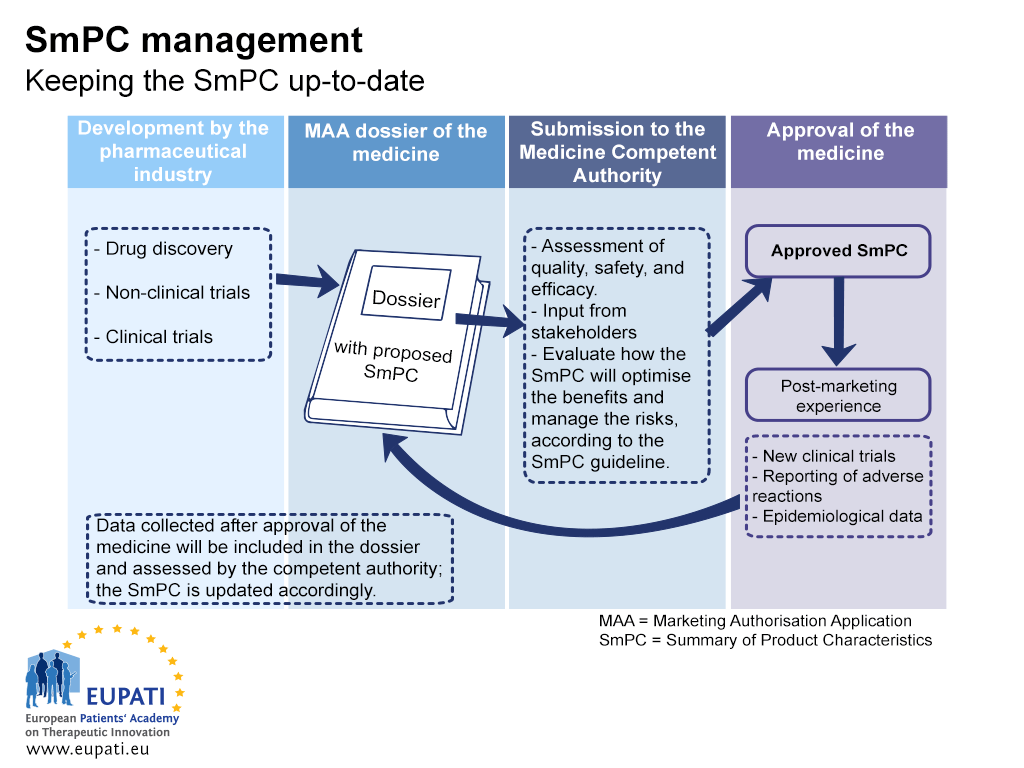
European Medicines Agency (EMA) approval processes for originator and... | Download Scientific Diagram