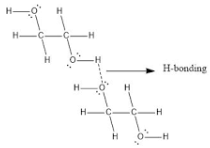
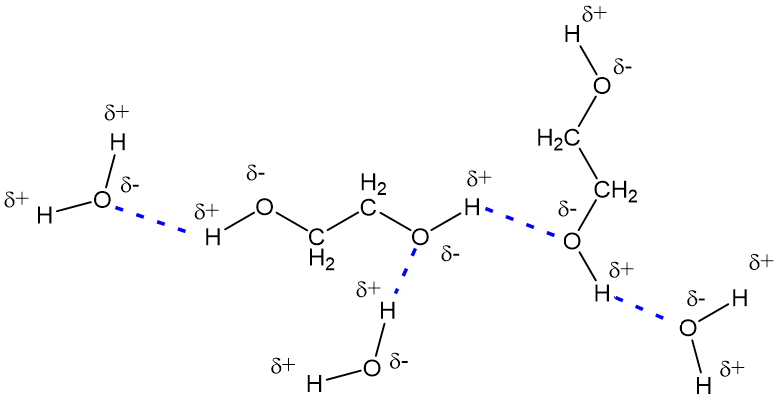
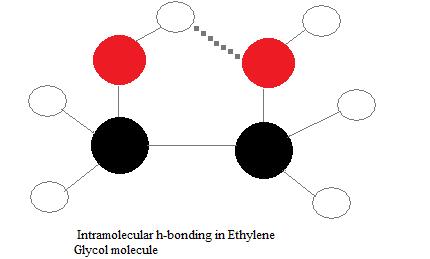
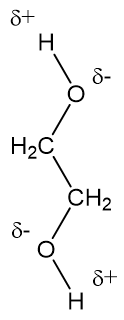
Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com

Hydrogen Bonds and Conformations in Ethylene Glycol under Pressure | The Journal of Physical Chemistry B

Schematic diagram of the two most stable conformers of (a) ethylene... | Download Scientific Diagram

Contact angles of water, diiodomethane, and ethylene glycol (a) and... | Download Scientific Diagram

10.12 | The molecular mass of butanol, C4H9OH, is 74.14; that of ethylene glycol, CH2(OH)CH2OH, is - YouTube

In the Lewis Dot structure of ethylene glycol, would one hydrogen atom visibly connect the two oxygen atoms? And if so, would one line connecting that same hydrogen atom to one of

Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com
![College Level: Inorganic Chemistry] Intermolecular Forces: Determining the solubility of Acetone/Urea in both Ethylene Glycol and O-Dichlorobenzene : r/HomeworkHelp College Level: Inorganic Chemistry] Intermolecular Forces: Determining the solubility of Acetone/Urea in both Ethylene Glycol and O-Dichlorobenzene : r/HomeworkHelp](https://i.redd.it/jdzmwtz099e41.jpg)