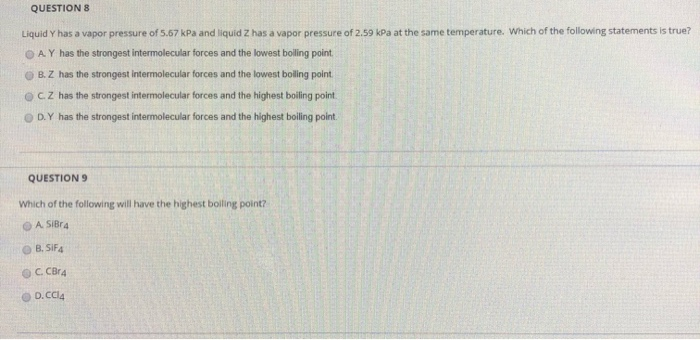
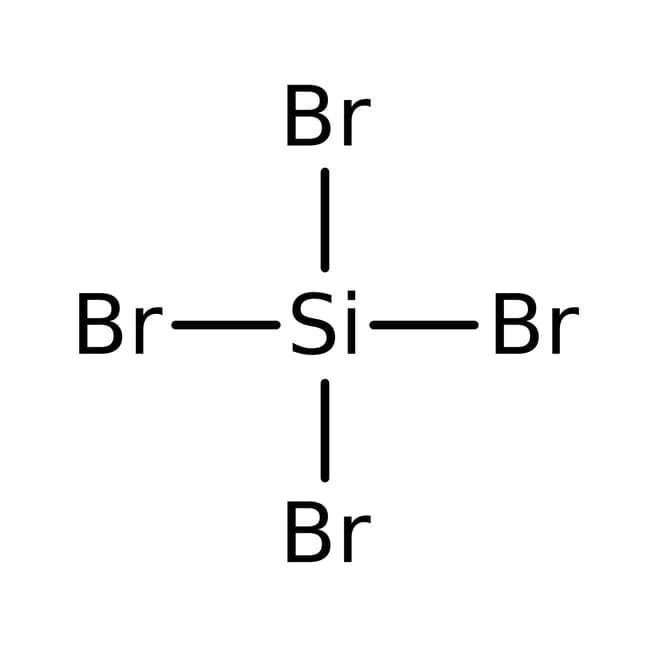SOLVED: QUESTION 5 The normal boiling points of two compe unds are tabulated below: Which of the following statements is TRUE for the higher boiling point of SiBr4 relative to NHz? gas

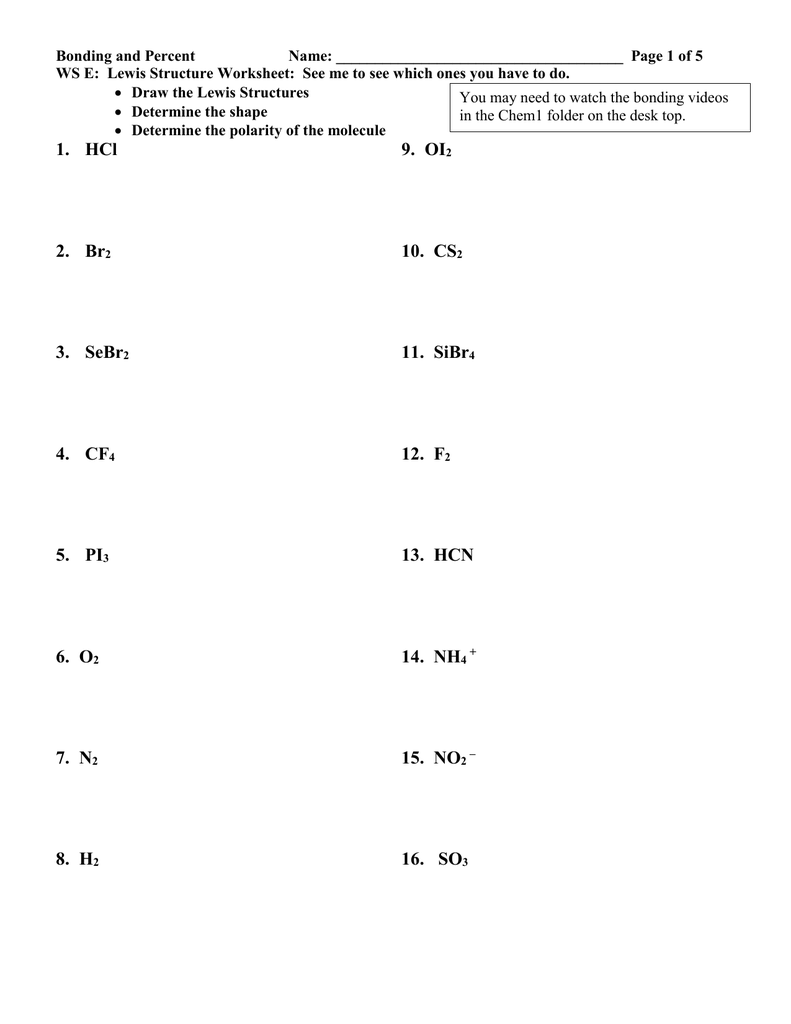
Bonding Click to start Question 1 Which compound contains ionic bonds? Ethanoic acid, CH 3 COOH Dichloroethane, CH 2 Cl 2 Silicon tetrabromide,SiBr ppt download
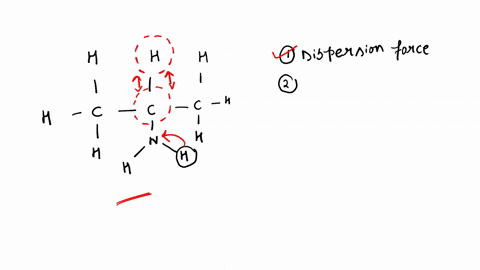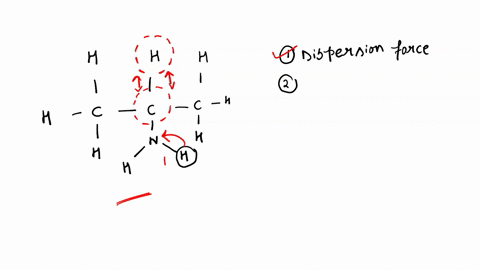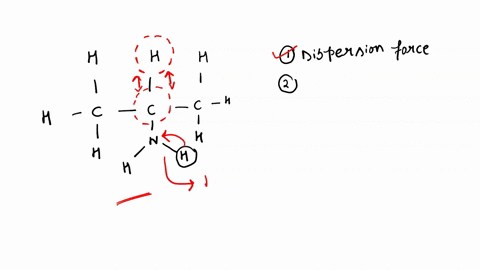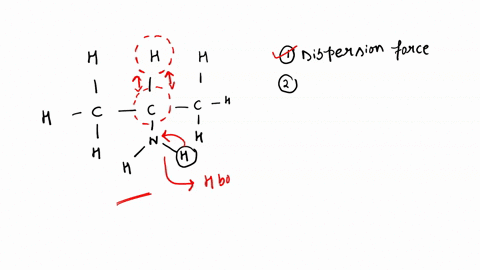
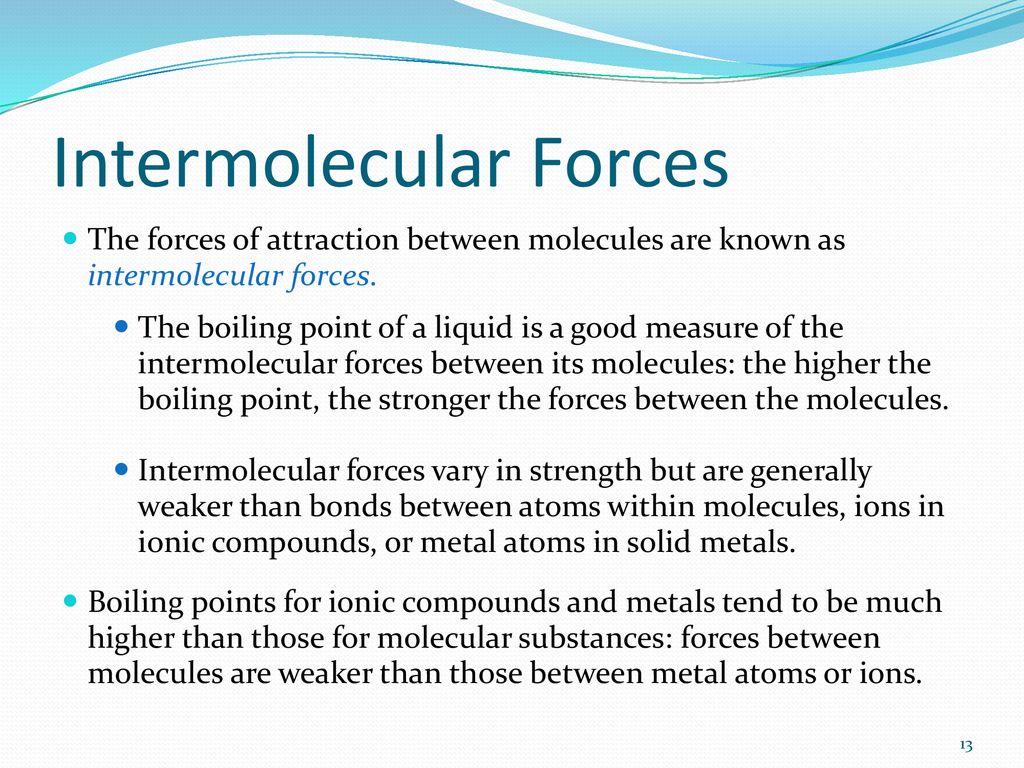
SOLVED: Identify the strongest type of intermolecular force acting between the given substances. Choose HB for hydrogen bond, DD for dipole-dipole, ID for ion-dipole, II for ion-induced dipole, DI for dipole-induced dipole

SOLVED: From the list of substances below, select those for which dipole-dipole forces contribute to the attraction between molecules in the gas or liquid phase: You may select more than one substance.
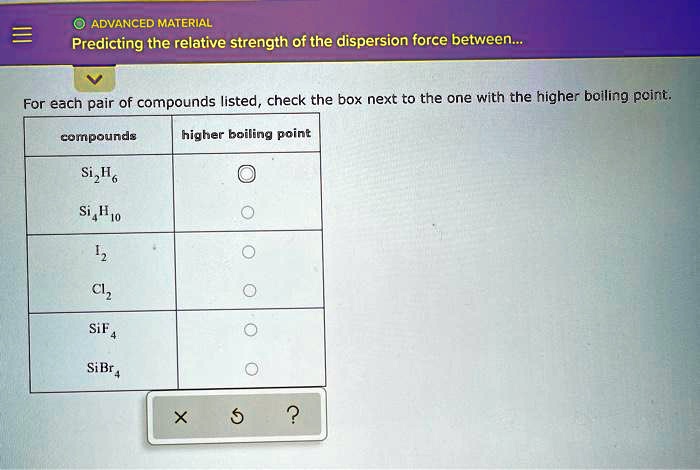
SOLVED: ADVANCED MATERIAL Predicting the relative strength of the dispersion force between For each pair of compounds listed check the box next to the one with the higher boiling pcint. compounds higher

SOLVED: '(a) The stronger hydrogen bonding force in SiBr4 contributes to its higher boiling point.(b) The stronger dipole-dipole interaction force in SiBr4 causes its higher boiling point.(c) The stronger London dispersion force

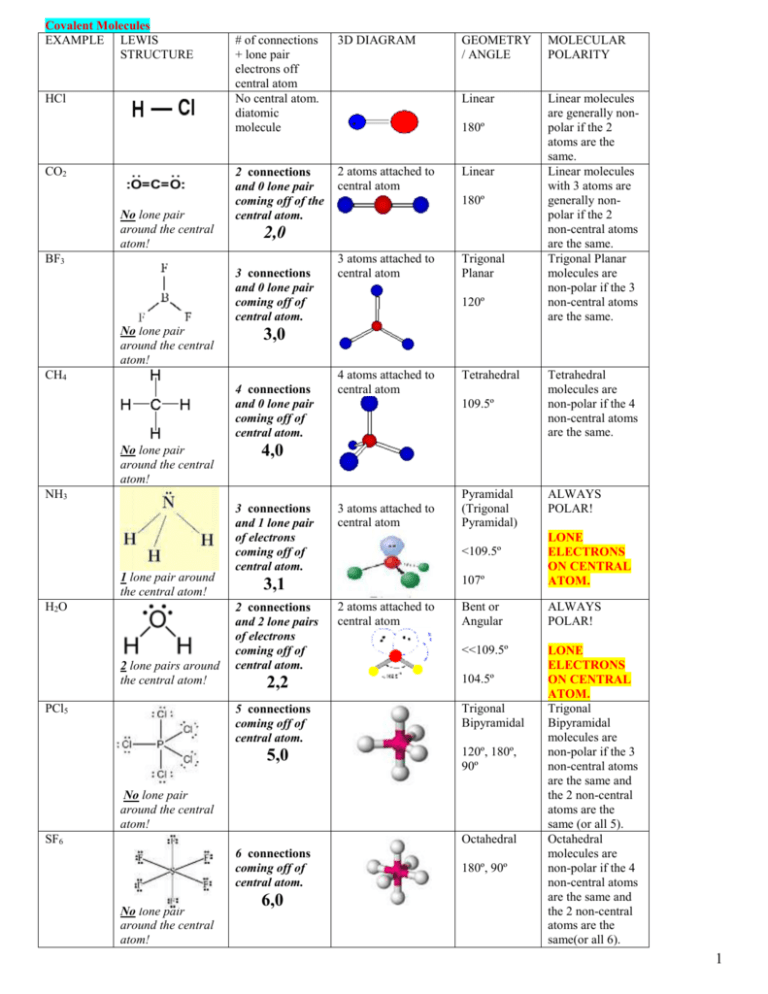
Sketch a microscopic picture of water and distinguish between intramolecular bonds and intermolecular forces. Which correspond to the bonds we draw in Lewis structures? | Homework.Study.com